
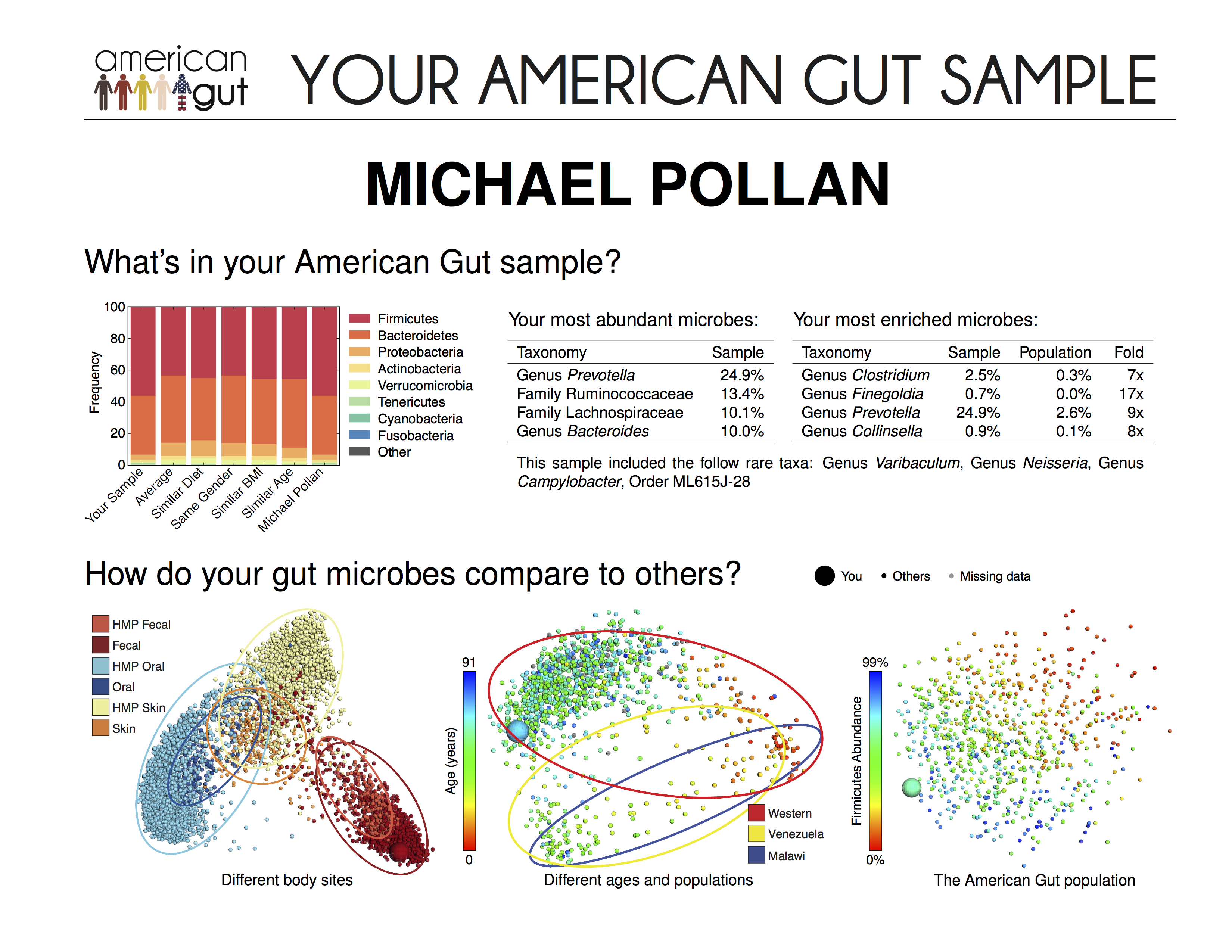
2015), the brain parenchyma is still considered “immune privileged” compared to peripheral tissues. Taken together, this suggests a critical role for the gut microbiota in regulating brain development and behavior, with the immune system emerging as an important coordinator of these interactions.ĭespite the recent discovery of meningeal lymphatics reshaping our understanding of immunity within the central nervous system (CNS) ( Louveau et al. Chronic inflammation not only changes the microbiota composition, but it can also trigger significant and long-lasting behavioral changes, such as the development of cognitive impairment and depression, in a bottom-up manner ( Lakhan and Kirchgessner 2010). The complexity of the microbiota is matched by the complexity of the host immune system to ensure the maintenance of this balance and for preventing access of organisms to the host inner milieu. In addition, there is increasing evidence that these microbial communities can regulate brain development, mood, and cognitive function through the bidirectional signaling between the gut and the brain ( Deverman and Patterson 2009 Lakhan and Kirchgessner 2010). 2004), as well as influencing the maturation and function of the intestinal immune system ( Caricilli et al. This complex community plays a critical role in the digestive process, protection against colonization with pathogens ( Sonnenburg et al. In mammals, the gastrointestinal (GI) tract harbors a complex microbial community, known as the intestinal microbiota, composed of thousands of different species of bacteria, viruses, fungi, archaea, and protists ( Parfrey et al. The intestine is the largest surface of the body and is constantly exposed to dietary and bacterial antigens as well as potentially noxious substances and infectious agents, which threaten the balance between health and disease ( Salvo Romero et al. Here we review the role of microbiota-immune interactions in the gut and the brain during homeostasis and disease and their impact on gut-brain communication, brain function, and behavior as well as the use of probiotics in central nervous system alterations. Consequently, probiotics, or beneficial microbes, are being recognized as promising therapeutic targets to modulate behavior and brain development by modulating the gut microbiota. Impairments in this bidirectional communication are implicated in the etiopathogenesis of psychiatric and neurodevelopmental diseases and disorders, including autism spectrum disorders, or comorbidities associated with Gastrointestinal diseases, including inflammatory bowel diseases, where dysbiosis is commonly seen. Activation of the immune system in the gut and in the brain are implicated in responses to neuroinflammation, brain injury, as well as changes in neurogenesis and plasticity. Gut bacteria can modulate not only gut-resident immune cells but also brain-resident immune cells. Numerous studies in recent years investigating the gut-brain axis have demonstrated an important role for the gut microbiota in modulating brain development and function, with the immune system serving as an important coordinator of these interactions. The complexity of the microbiota is matched by the complexity of the host immune system, where they have coevolved to maintain homeostasis ensuring the mutualistic host-microbial relationship. The vast diversity of bacteria that inhabit the gastrointestinal tract strongly influence host physiology, not only nutrient metabolism but also immune system development and function.


 0 kommentar(er)
0 kommentar(er)
